N-methyl-D-aspartate receptors (NMDARs) are glutamate-gated ion channels essential for excitatory neurotransmission, synaptic plasticity, and learning and memory. In a recent work published in Nature, a research team led by Prof. YU Jie at the Interdisciplinary Research Center on Biology and Chemistry, Shanghai Institute of Organic Chemistry, CAS, reported the structural basis underlying the diversity and activation mechanisms of native NMDARs directly purified from mouse brain tissue. In this work, the researchers directly purified endogenous NMDAR complexes from mouse whole-brain tissue and resolved ten distinct native receptor assemblies using a combination of subunit-specific antibody purification, single-molecule fluorescence imaging, and cryo-electron microscopy. The study reveals that GluN2A is the most prevalent subunit across native NMDAR populations and displays pronounced conformational flexibility in its amino-terminal domain. This intrinsic flexibility provides a structural explanation for the fast gating kinetics and dominant functional role of GluN2A-containing receptors in the brain. 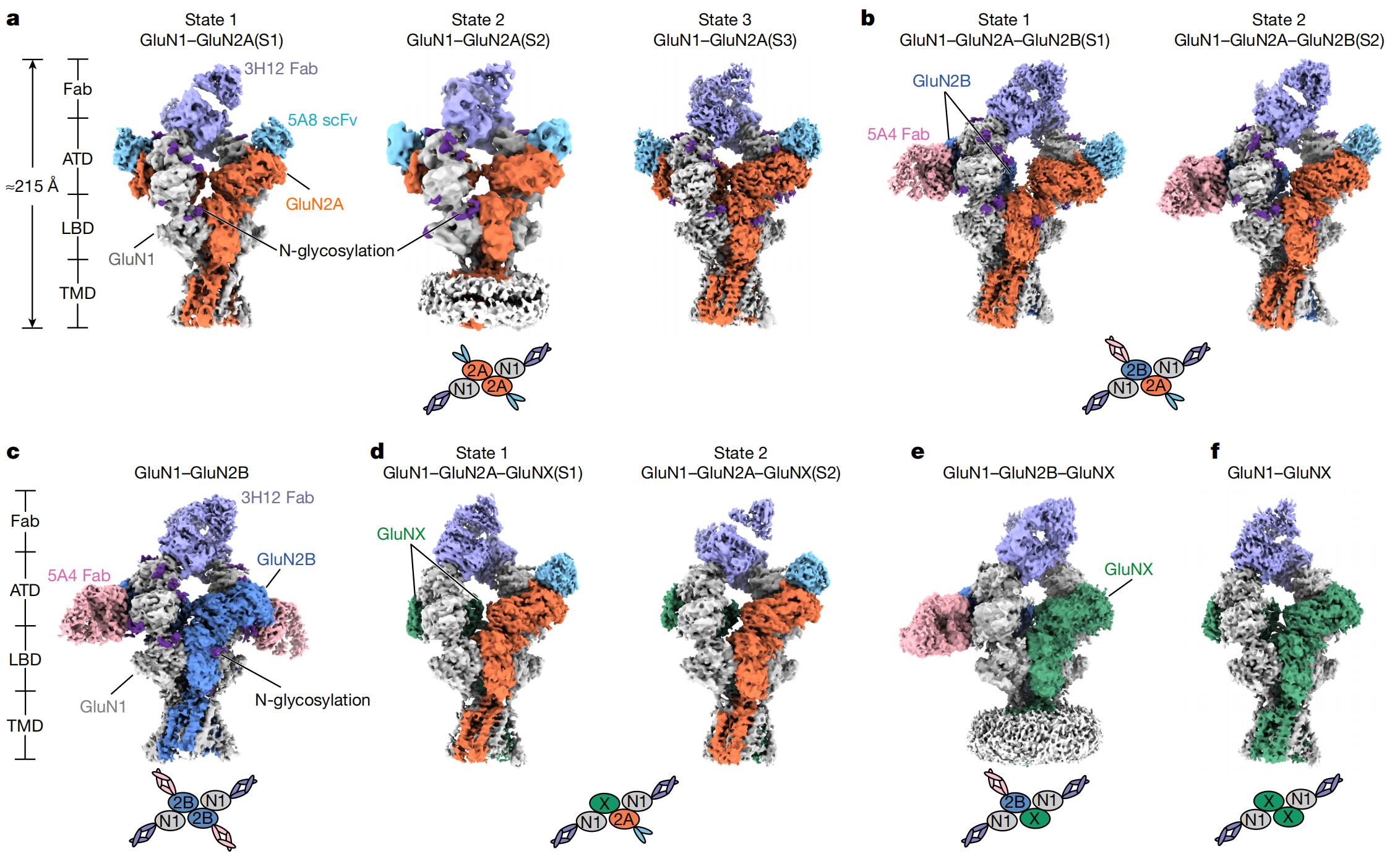
Figure. 1 Conformational diversity of native NMDA receptor structures (Image by YU Jie group) Importantly, the team captured a previously unseen fully open state of a native GluN1–GluN2B receptor. Unlike earlier structures that showed only partial pore dilation, the newly resolved structure demonstrates coordinated outward movements of the channel gate in both GluN1 and GluN2B subunits, resulting in a bona fide open ion-conducting pore. This finding resolves a long-standing question in the field regarding how co-agonist binding is translated into complete channel opening in NMDARs. By systematically mapping the composition, conformational diversity, and gating transitions of native NMDARs, this study provides a comprehensive structural framework for understanding excitatory synaptic signaling under physiological conditions. The results also establish a foundation for developing more precise therapeutic strategies targeting NMDAR dysfunction in brain disorders.
YU Jie Ph.D. Professor Interdisciplinary Research Center on Biology and Chemistry (IRCBC) Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences Haike Road 100 Shanghai 201204 China Email: yujie@sioc.ac.cn |


