Researchers from the Interdisciplinary Research Center on Biology and Chemistry at the Shanghai Institute of Organic Chemistry (SIOC), Chinese Academy of Sciences (CAS), led by Prof. YUAN Junying, have elucidated the regulatory mechanism of LOAD risk gene INPP5D in microglial RIPK1 activation, uncovering a new molecular link between neuroinflammation and late-onset Alzheimer’s disease (LOAD) with amyotrophic lateral sclerosis (ALS) comorbidity. The study is published in Immunity. INPP5D, a key genetic risk factor for LOAD, is highly expressed in microglia, yet its functional mechanism remained unclear. The team found that the N-terminal SH2 domain of INPP5D directly binds to the p-Y383 site of RIPK1, a serine/threonine kinase mediating neuroinflammation, to suppress its kinase activation—this process is independent of INPP5D’s phosphatase activity. Microglial INPP5D deficiency was shown to cell-autonomously activate RIPK1, driving the transcription of diverse proinflammatory mediators (including TLRs, inflammasomes and complement factors) and 17 LOAD risk genes such as ApoE and Trem2. In aging mice, myeloid INPP5D deficiency induced RIPK1-dependent dystrophic microglia, neuronal TDP-43 pathology, cortical neuron and motoneuron loss, as well as motor dysfunction in a non-cell-autonomous manner. Notably, RIPK1-regulated gene signatures in INPP5D-deficient murine microglia were highly consistent with lipid-processing and inflammatory microglial subtypes in human AD brains. In human iPSC-derived microglia carrying the AD-associated INPP5D risk allele, RIPK1 inhibition by Nec-1s effectively suppressed the proinflammatory response and downregulated AD risk genes, mirroring the results in mouse models. Genetic or pharmacological inhibition of RIPK1 reversed the above pathological changes in mice and human microglia, confirming RIPK1 as a downstream effector of INPP5D. This study identifies INPP5D as an intracellular rheostat controlling RIPK1-mediated neuroinflammation, and highlights RIPK1 kinase as a promising therapeutic target for LOAD and AD-ALS comorbidity. 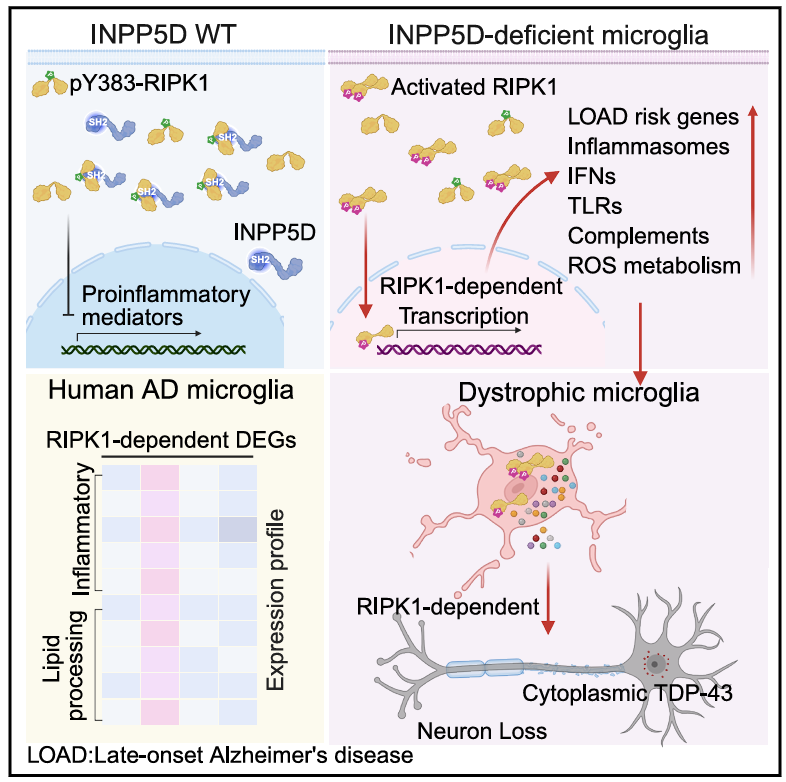
Figure 1. Working model of INPP5D in restraining RIPK1-mediated microglial inflammation and Alzheimer’s disease pathogenesis.
YUAN Junying, Ph.D, Professor Interdisciplinary Research Center on Biology and Chemistry (IRCBC) Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences Haike Road 100 Shanghai 201204 China Tel: 0086-21-68582313 Email: junying_yuan@sioc.ac.cn |


