Optically pure alcohols are prevalent motifs in natural products and pharmaceuticals, driving significant efforts to develop effective methods for their synthesis. Since the first reports in K-S (Kharasch-Sosnovsky) reaction in 1958, the copper-catalyzed radical-mediated sp3 C-H oxidation has emerged as an important method for the selective oxidation of allylic, propargylic, and other sp3 C–H bonds.In the past several decades, substantial progress has been made in developing enantioselective K-S reactions. Despite these advancements, however, most reported reactions suffer from limitations such as low-to-moderate enantioselectivities, poor reactivities, and the need for large excesses of cyclic alkenes (typically 5–10 equivalents), not to mention the site- and enantioselective sp3 C-H oxidation toward substrates bearing multiple similar C–H bonds. Organic chemists have been striving for more efficient and selective methods for over 60 years, yet a general solution that balances both efficiency and selectivity remains elusive. Recently, a research paper titled“Site- and enantioselective allylic and propargylic C–H oxidation enabled by copper-based biomimetic catalysis” was published on the Nature catalysis journal by Professor LIN Zhenyang at Hongkong University of Science and Technology and LIU Guosheng at Shanghai Institute of Organic Chemistry (Nat. Catal. DOI:10.1038/s41929-024-01276-4). In this paper, they described how a copper-bound tert-butoxy radical intermediate facilitates site- and enantio-selectively oxidation of C(sp3)-H on a range of alkenes and alkynes. This method efficiently marches forth without large excess of C-H substrates, allowing synthetic community could modify the oxidation state at targeting site on complex molecules, more efficiently and more precisely. 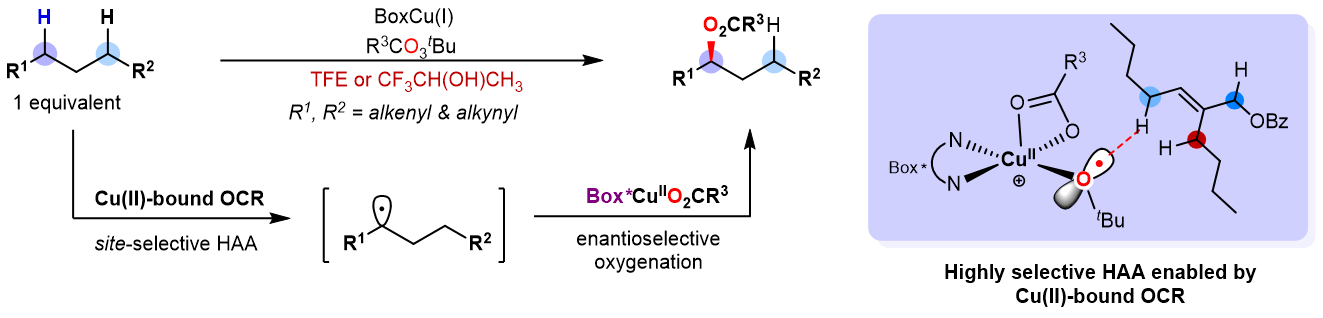
Figure 1. Site- and enantioselective allylic and propargylic C(sp3)-H oxidation enabled by Cu(II)-bound OCR (Image by LIU Guosheng) Researchers from LIN and LIU’s lab have discovered that employing fluoro-containing alcohols (e.g., trifluoroethanol, TFE) as solvent is crucial for the significant enhancement of reaction efficiency and selectivity. Most important, an unprecedent copper-bound tert-butoxy radical (Cu(II)-bound OCR) was proposed as a key intermediate for the hydrogen atom abstraction (HAA), which behaves like the active site of a copper-based enzyme, both structurally and functionally. Professor LIU explained that, “the chiral and bulky environment built by ligand has endowed this copper-bound tert-butoxy radical with the increased HAA ability as well as the capabilities to precisely distinguish similar C-H bonds.” In addition, compared to the predominantly quenching process of free tert-butoxy radical by the Cu(I)-catalyst, their developed Cu(II)-bound OCR system undergoes effective HAA process owing to the bulky steric effect, which enables the highly efficient C-H oxidation with C-H substrates as limiting reagent. This approach demonstrates an impressively broad substrate scope, highlighting its utility and effectiveness. Alkenes and alkynes bearing wide array of functional groups could be readily transformed into targeting enantiomeric-enriched esters with good yields and excellent enantioselectivities. Notably, bioactive molecules, including natural products and drugs, could also be oxidized with remarkable site-selectivity, further underscoring the promise of this strategy. 
Figure 2. Selected examples enabled by copper-based biomimetic strategy (Image by LIU Guosheng)
LIU Guosheng Ph.D. Professor Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences Ling Ling Road 345 Shanghai 200032 China Tel: 0086-21-54925346 Email: gliu@mail.sioc.ac.cn |


